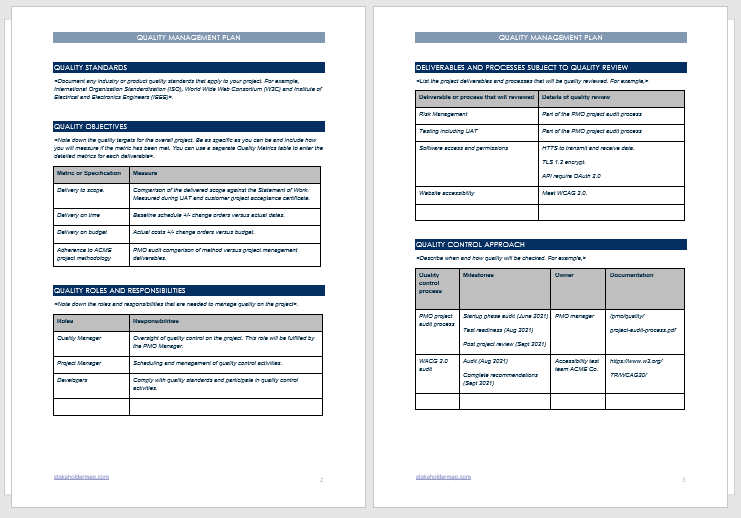
So, you’re tasked with setting up a Quality Management System (QMS)? That’s fantastic! A well-implemented QMS can streamline your processes, improve product quality, boost customer satisfaction, and ultimately, make your business more successful. But where do you even begin? One of the biggest hurdles is often the documentation – creating and maintaining the essential records that demonstrate your commitment to quality. Don’t worry, it doesn’t have to be a daunting task. That’s where a quality management system documentation template comes in handy. It’s your starting point, your framework, and your guide to building a robust and effective QMS.
Think of a quality management system documentation template as a blueprint for your QMS. It provides a structure and predefined formats for documenting your policies, procedures, processes, and records. This not only saves you a significant amount of time and effort but also ensures consistency and compliance with relevant standards, such as ISO 9001. It’s like having a ready-made foundation upon which you can build your quality house, customized to perfectly fit your organization’s unique needs and goals.
In this article, we’ll dive deeper into the world of quality management system documentation. We’ll explore the different types of documents you’ll need, why they’re so important, and how to choose the right template to get you started. We will also discuss how to customize the template and maintain the documentations after. Get ready to transform your QMS documentation from a source of stress into a valuable asset for your business.
Why is QMS Documentation So Important?
You might be wondering, “Why all the fuss about documentation?” It’s a valid question. After all, spending time writing things down can feel like a distraction from the actual work. However, QMS documentation is far from just paperwork; it’s the backbone of your quality system. Think of it as the memory of your organization, preserving knowledge and best practices to ensure consistent quality, no matter who’s involved. Without proper documentation, your QMS is like a ship without a rudder, prone to drifting off course.
Firstly, documentation provides evidence. It demonstrates that your organization is following established procedures and meeting required standards. This is crucial for audits, certifications, and regulatory compliance. A well-documented QMS shows that you’re taking quality seriously and are committed to continuous improvement.
Secondly, documentation promotes consistency. By outlining specific processes and procedures, it reduces the likelihood of errors and variations. Everyone is on the same page, following the same guidelines, leading to more predictable and reliable outcomes. This is especially important in organizations with multiple departments or locations.
Thirdly, documentation facilitates training. New employees can quickly learn the ropes by referring to documented procedures and work instructions. This shortens the learning curve, reduces training costs, and ensures that everyone is performing their tasks correctly. It also serves as a valuable resource for existing employees who need a refresher or clarification on specific processes.
Finally, documentation enables continuous improvement. By tracking key metrics and documenting the results of audits and reviews, you can identify areas for improvement and implement corrective actions. This iterative process helps you to refine your processes, reduce waste, and enhance the overall effectiveness of your QMS.
Common types of documents
You might be wondering what kind of documentations are generally used in a QMS. The followings are some of the commonly used documentations that can be found in a QMS.
- Quality Manual: A high-level document that outlines your organization’s quality policy and objectives.
- Procedures: Detailed instructions on how to perform specific tasks or processes.
- Work Instructions: Step-by-step guides for completing specific tasks.
- Forms and Templates: Standardized documents for recording data and information.
- Records: Evidence that processes have been carried out according to documented procedures.
Choosing and Using a Quality Management System Documentation Template
Now that you understand the importance of QMS documentation, let’s talk about choosing and using a quality management system documentation template. With so many options available online, it’s important to select one that aligns with your organization’s specific needs and goals. Start by considering your industry, the size of your company, and the specific requirements of any relevant standards or regulations. A generic template might not be sufficient; you’ll likely need to customize it to reflect your unique processes and terminology.
Look for a template that is well-structured, easy to understand, and customizable. It should include all the essential documents you’ll need to build a comprehensive QMS, such as a quality manual template, procedure templates, and form templates. The template should also be compatible with your organization’s existing software and systems. Consider the file format (Word, Excel, PDF) and whether it can be easily integrated into your document management system.
Once you’ve selected a template, it’s time to start customizing it. This is where you tailor the template to reflect your organization’s specific processes, procedures, and terminology. Don’t just blindly fill in the blanks; take the time to understand each section and adapt it to your unique needs. Involve key stakeholders from different departments to ensure that the documentation accurately reflects the way things are actually done in your organization.
As you customize the template, pay attention to clarity and conciseness. Use clear, simple language that everyone can understand. Avoid jargon and technical terms unless they are essential. Use visuals, such as flowcharts and diagrams, to illustrate complex processes. Remember, the goal is to create documentation that is easy to use and understand.
Finally, don’t forget about version control. As you update and revise your documentation, it’s important to keep track of changes and maintain a history of previous versions. This will help you to avoid confusion and ensure that everyone is working with the most up-to-date information. Use a version control system to track changes, assign responsibility for updates, and archive previous versions.
Implementing a QMS and maintaining its documentation is a continuous journey, not a one-time event. By embracing a culture of quality and continuously seeking ways to improve your processes, you can create a QMS that truly adds value to your organization.
A quality management system documentation template is a great way to jumpstart your QMS implementation. Remember to customize it to your specific needs, and keep it updated. Embrace quality as a continual improvement process and your company will see the benefits.